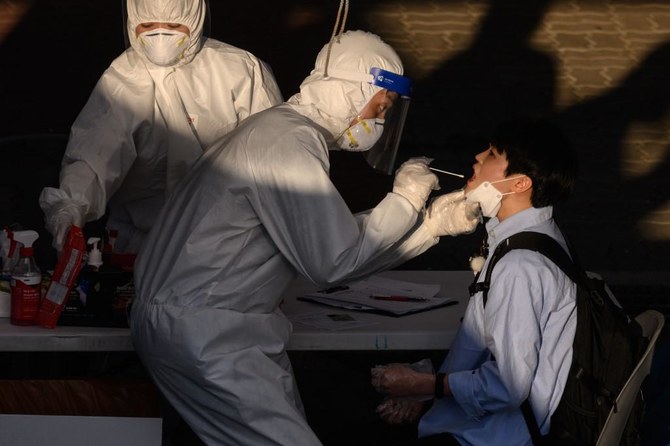
SEOUL: South Korea reported its biggest spike in coronavirus cases in nearly two months on Thursday, as officials scramble to tackle fresh clusters that have raised concerns of a possible second wave of infections.
The country has been held up as a global model in how to curb the virus and has begun to ease restrictions, but is now rushing to contain new infections as life returns to normal.
Officials announced 79 new cases Thursday — taking its total to 11,344 — with most fresh infections from the densely populated Seoul metropolitan area.
It was the largest increase since 81 cases were announced on April 5.
An outbreak at a warehouse of e-commerce firm Coupang in Bucheon, west of Seoul, has seen 69 cases, said the Korea Centers for Disease Control and Prevention.
Around 4,100 workers and visitors to the building were under self-isolation, with more than 80 percent tested so far, vice health minister Kim Gang-lip told reporters.
“We are expecting the number of new cases linked to the warehouse to continue rising until today as we wrap up related tests,” he added.
Social distancing rules have been relaxed in South Korea and facilities such as museums and churches have reopened. Some professional sports — including baseball and soccer — started new seasons earlier this month, albeit behind closed doors.
Students have been returning to classes since last week, although some schools were forced to turn away their pupils over concerns of new virus cases in their neighborhood.
The country endured one of the worst early outbreaks of the disease outside mainland China, and while it never imposed a compulsory lockdown, strict social distancing had been widely observed since March.
But it appears to have brought its outbreak under control thanks to an extensive “trace, test and treat” program.


South Korea sees largest spike in virus cases in nearly two months
Short Url
https://arab.news/jwpvv
South Korea sees largest spike in virus cases in nearly two months

- Officials announced 79 new cases on Thursday
- It was the largest increase since April 5
Kim unveils homes for kin of North Korean troops killed aiding Russia: KCNA
SEOUL: North Korean leader Kim Jong Un touted a newly built street of flats for families of soldiers killed supporting Russia’s war against Ukraine, state media reported Monday, with photos showing him accompanied by his daughter.
North Korea has deployed thousands of troops to fight for Russia, according to South Korean and Western intelligence agencies, and Seoul has estimated that around 2,000 have been killed.
Analysts say North Korea is receiving financial aid, military technology and food and energy supplies from Russia in return.
“The new street has been built thanks to the ardent desire of our motherland that wishes that... its excellent sons, who defended the most sacred things by sacrificing their most valuable things, will live forever,” Kim said in a speech released by the official Korean Central News Agency.
The report on Monday did not mention Russia, but Kim last week pledged to “unconditionally support” all of Russian President Vladimir Putin’s policies and decisions.
“Before their death, the heroic martyrs must have pictured in their mind’s eye their dear families living in the ever-prospering country,” he added.
Photos released by KCNA show Kim touring the new homes built for the families on Saeppyol Street, alongside his teenage daughter Ju Ae, widely viewed as his heir apparent.
Seoul’s spy agency said last week she had now been clearly “designated as a successor,” citing her participation in high-profile events with her father.
One photo shows Kim speaking with what appeared to be the family members of a fallen soldier on a sofa, his daughter standing behind them.
Other photos show families checking the utilities in their new flats.
The rollout comes ahead of Pyongyang’s biggest political event on the calendar — the party congress — scheduled to take place later this month, although the exact date has not been announced.
Attention is on which foreign and domestic policy directions Kim will declare to set the country’s course, as well as whether Ju Ae will be given any official party titles.
The timing of the street inauguration is a “highly calculated political move to justify its soldier deployment” ahead of the party congress, Hong Min, an analyst at the Korea Institute for National Unification, told AFP.
“It visualizes the state providing concrete compensation to the families of fallen soldiers... as a symbolic showcase,” he said.
North Korea has deployed thousands of troops to fight for Russia, according to South Korean and Western intelligence agencies, and Seoul has estimated that around 2,000 have been killed.
Analysts say North Korea is receiving financial aid, military technology and food and energy supplies from Russia in return.
“The new street has been built thanks to the ardent desire of our motherland that wishes that... its excellent sons, who defended the most sacred things by sacrificing their most valuable things, will live forever,” Kim said in a speech released by the official Korean Central News Agency.
The report on Monday did not mention Russia, but Kim last week pledged to “unconditionally support” all of Russian President Vladimir Putin’s policies and decisions.
“Before their death, the heroic martyrs must have pictured in their mind’s eye their dear families living in the ever-prospering country,” he added.
Photos released by KCNA show Kim touring the new homes built for the families on Saeppyol Street, alongside his teenage daughter Ju Ae, widely viewed as his heir apparent.
Seoul’s spy agency said last week she had now been clearly “designated as a successor,” citing her participation in high-profile events with her father.
One photo shows Kim speaking with what appeared to be the family members of a fallen soldier on a sofa, his daughter standing behind them.
Other photos show families checking the utilities in their new flats.
The rollout comes ahead of Pyongyang’s biggest political event on the calendar — the party congress — scheduled to take place later this month, although the exact date has not been announced.
Attention is on which foreign and domestic policy directions Kim will declare to set the country’s course, as well as whether Ju Ae will be given any official party titles.
The timing of the street inauguration is a “highly calculated political move to justify its soldier deployment” ahead of the party congress, Hong Min, an analyst at the Korea Institute for National Unification, told AFP.
“It visualizes the state providing concrete compensation to the families of fallen soldiers... as a symbolic showcase,” he said.
© 2026 SAUDI RESEARCH & PUBLISHING COMPANY, All Rights Reserved And subject to Terms of Use Agreement.