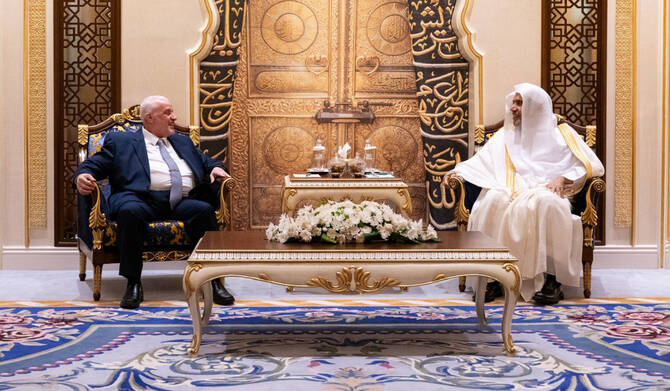
RIYADH: Dr. Mohammed bin Abdulkarim Al-Issa, secretary-general of the Muslim World League and chairman of the Organization of Muslim Scholars, met with the Syrian Arab Republic’s Minister of Awqaf Mohammad Abu Al-hair Shukri to discuss “a variety of topics of common interest,” the MWL wrote in a post on X on Saturday.
Meanwhile, Saudi Minister of Islamic Affairs, Dawah and Guidance Dr. Abdullatif Al-Alsheikh recently met with the Malaysian delegation of Islamic leaders participating in the Custodian of the Two Holy Mosques Guests Program for Hajj, Umrah, and Visit, the Saudi Press Agency reported on Saturday.